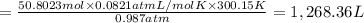Chemistry, 19.10.2019 00:10 jimperez9616
During world war ii, a portable source of hydrogen gas was needed for weather balloons, and solid metal hydrides were the most convenient form. many metal hydrides react with water to generate the metal hydroxide and hydrogen. two candidates were lithium hydride and magnesium hydride. what volume of gas is formed from 1.40 lb of each hydride at 750. torr and 27°c?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
During world war ii, a portable source of hydrogen gas was needed for weather balloons, and solid me...
Questions


Social Studies, 16.11.2020 20:40


Biology, 16.11.2020 20:40




Chemistry, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40

History, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40


Mathematics, 16.11.2020 20:40


English, 16.11.2020 20:40

Spanish, 16.11.2020 20:40

History, 16.11.2020 20:40

Arts, 16.11.2020 20:40

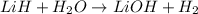
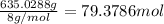
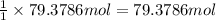 of hydrogen gas.
of hydrogen gas.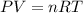
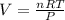
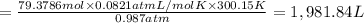
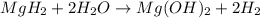
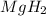 = 1.40 lb = 635.0288 g
= 1.40 lb = 635.0288 g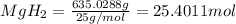
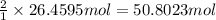 of hydrogen gas.
of hydrogen gas.