
Chemistry, 19.10.2019 00:20 alexvillaa121
Ph is a logarithmic scale used to indicate the hydrogen ion concentration, [h+], of a solution: ph=−log[h+] due to the autoionization of water, in any aqueous solution, the hydrogen ion concentration and the hydroxide ion concentration, [oh−], are related to each other by the kw of water: kw=[h+][oh−]=1.00×10^−14 where 1.00×10^−14 is the value at approximately 297 k. based on this relation, the ph and poh are also related to each other as 14.00=ph+poh. the temperature for each solution is carried out at approximately 297 k where kw=1.00×10^−14. part a) 0.35 g of hydrogen chloride (hcl) is dissolved in water to make 2.5 l of solution. what is the ph of the resulting hydrochloric acid solution? express the ph numerically to two decimal places.
part b) 0.80 g of sodium hydroxide (naoh) pellets are dissolved in water to make 2.0 l of solution. what is the ph of this solution?
express the ph numerically to two decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2


Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

You know the right answer?
Ph is a logarithmic scale used to indicate the hydrogen ion concentration, [h+], of a solution: ph=...
Questions


Mathematics, 23.04.2020 23:49




Biology, 23.04.2020 23:49




Business, 23.04.2020 23:49



Computers and Technology, 23.04.2020 23:49


Social Studies, 23.04.2020 23:49

Mathematics, 23.04.2020 23:49

Biology, 23.04.2020 23:49



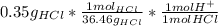 = 9.60*10⁻³ mol H⁺
= 9.60*10⁻³ mol H⁺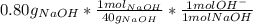 = 0.02 mol OH⁻
= 0.02 mol OH⁻

