
Chemistry, 19.10.2019 01:00 adiaripley6408
The energy of an electrostatic interaction between two charged atoms is dependent on the charges on the atoms, the distance between them, and the dielectric constant of the solvent. for example, the strength of a weak acid (ka, acid dissociation constant) depends on the strength of the electrostatic interaction between a negatively charged carboxylic acid group and a proton. the solvent dielectric constant has a large influence on the pka for weak acids. select the statements that correctly explain the influence of two solvents, water and hexane, on the pka of acetic acid.
in water, the association of ch3coo– and h will be favored because charge-charge interactions are stronger in water.
nonpolar solvents like hexane will weaken charge-charge interactions resulting in more dissociation of h .
the pka will be higher in hexane.
hexane cannot affect the pka of acetic acid since hexane cannot donate or accept h .
more h will dissociate in water because the dielectric constant is higher.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

You know the right answer?
The energy of an electrostatic interaction between two charged atoms is dependent on the charges on...
Questions

Mathematics, 16.12.2021 01:00


History, 16.12.2021 01:00


Computers and Technology, 16.12.2021 01:00



History, 16.12.2021 01:00



English, 16.12.2021 01:00






Mathematics, 16.12.2021 01:00

Mathematics, 16.12.2021 01:00

Social Studies, 16.12.2021 01:00

Mathematics, 16.12.2021 01:00

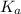 will be low , hence
will be low , hence 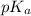 will be high.
will be high. 

