
Chemistry, 22.10.2019 18:50 babyrocks7300
Strike anywhere matches contain the compound tetraphosphorus trisulfide, which burns to form tetraphosphorus decaoxide and sulfur dioxide gas. how many milliliters of sulfur dioxide, measured at 747 torr and 23.8°c, can be produced from burning 0.576 g of tetraphosphorus trisulfide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3


Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2

You know the right answer?
Strike anywhere matches contain the compound tetraphosphorus trisulfide, which burns to form tetraph...
Questions

History, 03.07.2019 21:00



English, 03.07.2019 21:00

History, 03.07.2019 21:00


Physics, 03.07.2019 21:00

Mathematics, 03.07.2019 21:00

Business, 03.07.2019 21:00


Mathematics, 03.07.2019 21:00

History, 03.07.2019 21:00


Mathematics, 03.07.2019 21:00

Mathematics, 03.07.2019 21:00


English, 03.07.2019 21:00

Chemistry, 03.07.2019 21:00


Mathematics, 03.07.2019 21:00

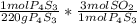 = 7.85 * 10⁻³ mol SO₂ = n
= 7.85 * 10⁻³ mol SO₂ = n

