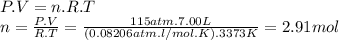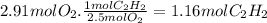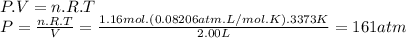Chemistry, 22.10.2019 23:00 kihgff5711
Magine that you have a 7.00 l gas tank and a 2.00 l gas tank. you need to fill one tank with oxygen and the other with acetylene to use in conjunction with your welding torch. if you fill the larger tank with oxygen to a pressure of 115 atm , to what pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? assume ideal behavior for all gases.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3


Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Magine that you have a 7.00 l gas tank and a 2.00 l gas tank. you need to fill one tank with oxygen...
Questions


English, 30.11.2021 21:20

Business, 30.11.2021 21:20






Mathematics, 30.11.2021 21:20

Mathematics, 30.11.2021 21:20


Mathematics, 30.11.2021 21:20






Biology, 30.11.2021 21:20