
Chemistry, 23.10.2019 01:00 little68941
Enter your answer in the provided box. roasting galena [lead(ii) sulfide] is an early step in the industrial isolation of lead. how many liters of sulfur dioxide, measured at stp, are produced by the reaction of 5.05 kg of galena with 123 l of oxygen gas at 220°c and 2.00 atm

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 08:10
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1
You know the right answer?
Enter your answer in the provided box. roasting galena [lead(ii) sulfide] is an early step in the in...
Questions

Mathematics, 30.09.2021 21:00





Mathematics, 30.09.2021 21:10

Biology, 30.09.2021 21:10

Mathematics, 30.09.2021 21:10

Mathematics, 30.09.2021 21:10




Arts, 30.09.2021 21:10

Mathematics, 30.09.2021 21:10


Computers and Technology, 30.09.2021 21:10




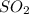 produced are 90.7 liters.
produced are 90.7 liters.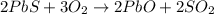
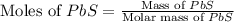
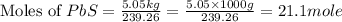
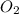 by using ideal gas equation.
by using ideal gas equation.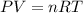
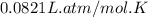
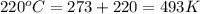
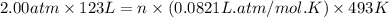
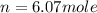
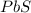
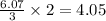 moles of
moles of 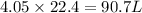 volume of
volume of 

