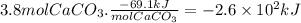Chemistry, 23.10.2019 18:00 noahdavis4650
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancient romans as mortar in stone structures. the reaction for this process is
ca(oh)2(s) + co2(g) --> caco3(s) + h2o(g) ; δh = -69.1 kj
what is the enthalpy change if 3.8 mol of calcium carbonate is formed?
(a) -18 kj
(b) -69 kj
(c) -73 kj
(d) -260 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancie...
Questions