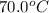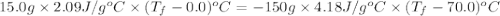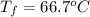15.0 g of ice cubes at 0.0°c are combined with 150. g of liquid water at 70.0°c in a coffee cup calorimeter. calculate the final temperature reached, assuming no heat loss or gain from the surroundings. (the specific heat capacity of h2o(l), cs = 4.18 j/gc; h2o(s) h2o(l) h = 6.02 kj/mol)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
15.0 g of ice cubes at 0.0°c are combined with 150. g of liquid water at 70.0°c in a coffee cup calo...
Questions




Geography, 22.08.2019 18:10

History, 22.08.2019 18:10


Biology, 22.08.2019 18:10

Biology, 22.08.2019 18:10




Biology, 22.08.2019 18:10





Mathematics, 22.08.2019 18:10

Computers and Technology, 22.08.2019 18:10

Computers and Technology, 22.08.2019 18:10

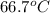
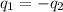
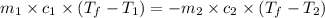
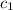 = specific heat of ice =
= specific heat of ice = 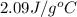
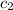 = specific heat of water =
= specific heat of water = 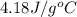
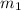 = mass of ice = 15.0 g
= mass of ice = 15.0 g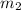 = mass of water = 150 g
= mass of water = 150 g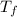 = final temperature = ?
= final temperature = ?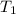 = initial temperature of ice =
= initial temperature of ice = 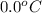
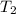 = initial temperature of water =
= initial temperature of water =