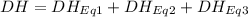At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj eq. 2: 2 o3(g) → 3 o2(g) δh = –284.6 kj eq. 3: 2 o2(g) → 4 o(g) δh = –990.0 kj use the above data to calculate the heat absorbed (kj) when 2.5 moles of no2(g) is formed at room temperature according to the chemical reaction. do not include units. no(g) + o(g) → no2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2...
Questions

Health, 16.11.2020 17:20



Mathematics, 16.11.2020 17:20


Arts, 16.11.2020 17:20


Mathematics, 16.11.2020 17:20


Social Studies, 16.11.2020 17:20



Spanish, 16.11.2020 17:20

Mathematics, 16.11.2020 17:20

Biology, 16.11.2020 17:20


History, 16.11.2020 17:20

Biology, 16.11.2020 17:20