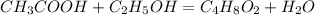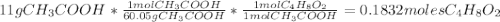Determine the theoretical maximum moles of ethyl acetate, , that could be produced in this experiment. the reactant, acetic acid, is the limiting reagent. (to avoid introducing rounding errors on intermediate calculations, enter your answer to four significant figures.) theoretical maximum moles of ethyl acetate = mol reactant mass 11.0 g product mass 10.8 g reactant moles 0.1833 mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
Determine the theoretical maximum moles of ethyl acetate, , that could be produced in this experimen...
Questions







Business, 21.02.2020 20:54





Mathematics, 21.02.2020 20:54







Mathematics, 21.02.2020 20:55

Geography, 21.02.2020 20:56

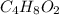 )
)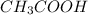 reacts with ethanol to produce ethyl acetate
reacts with ethanol to produce ethyl acetate