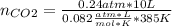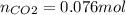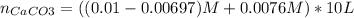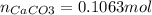Chemistry, 05.11.2019 02:31 adriandehoyos1p3hpwc
Enter your answer in the provided box. when 0.100 mol of caco3(s) and 0.100 mol of cao(s) are placed in an evacuated, sealed 10.0−l container and heated to 385 k, pco2 = 0.220 atm after equilibrium is established: caco3(s) ⇌ cao(s) + co2(g) an additional 0.240 atm of co2(g) is pumped in. what is the total mass (in g) of caco3 after equilibrium is reestablished?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 23.06.2019 11:30
How many grams of carbon are in 237 grams of ethanol(c2h5oh) and how many sulfide ions are in 2.45 moles of aluminum sulfide show me you you got the answers
Answers: 3

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
You know the right answer?
Enter your answer in the provided box. when 0.100 mol of caco3(s) and 0.100 mol of cao(s) are placed...
Questions


Computers and Technology, 08.03.2021 19:50



History, 08.03.2021 19:50



English, 08.03.2021 19:50


History, 08.03.2021 19:50

Mathematics, 08.03.2021 19:50


Mathematics, 08.03.2021 19:50






English, 08.03.2021 19:50

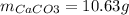
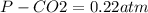
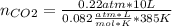
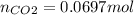
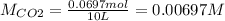
![K=[CO_2]](/tpl/images/0359/7950/b2036.png)