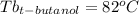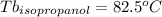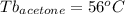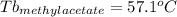Chemistry, 05.11.2019 02:31 Tweektweak
Astudent is given an unknown, clear, colorless liquid at room temperature. the student measures the density, melting point, and boiling point of the liquid. what would the student conclude if he or she found out that the unknown had a density of .79 g/cm3 and a boiling point is 82.05°c? a the substance is t-butanol b the substance is isopropanol c the substance is acetone d the substance is methyl acetate

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Astudent is given an unknown, clear, colorless liquid at room temperature. the student measures the...
Questions




Computers and Technology, 30.09.2019 22:30










Computers and Technology, 30.09.2019 22:30