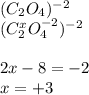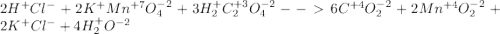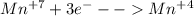Chemistry, 08.11.2019 01:31 JamesLachoneus
In an ion, the sum of the oxidation states is equal to the overall ionic charge. note that the sign of the oxidation states and the number of atoms associated with each oxidation state must be considered. in oh-, for example, the oxygen atom has an oxidation state of 2 and the hydrogen atom has an oxidation state of +1, for a total of ( -2 ) + ( +1 ) = -1
(i) what is the oxidation state of each individual carbon atom in c2o4 2^-
express the oxidation state numerically (e. g., +1).
(ii) which element is reduced in this reaction?
2hcl+ 2kmno4 + 3h2c2o4 ? 6co2 +2mno2 + 2kcl + 4h2o

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
In an ion, the sum of the oxidation states is equal to the overall ionic charge. note that the sign...
Questions

Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01



Chemistry, 01.07.2020 15:01

Biology, 01.07.2020 15:01




Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01



History, 01.07.2020 15:01

English, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01



Mathematics, 01.07.2020 15:01