
Chemistry, 09.11.2019 05:31 brooklynpage3930
The heats of combustion of ethane (c2h6) and butane (c4h10) are 52 kj/g and 49 kj/g, respectively. we need to produce 1.000 x 103 kj heat by burning one of the fuels. which fuel will emit the least amount of co2? 1. calculate the number of grams needed of each fuel: 2. calculate the number of moles of each fuel: 3. write down the balanced chemical equation for the combustion of the fuels: 4. calculate the number of moles of co2 produced by burning each fuel to produce 1.000 x 103 kj. which fuel will emit the least amount of co2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1


Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
The heats of combustion of ethane (c2h6) and butane (c4h10) are 52 kj/g and 49 kj/g, respectively. w...
Questions



Mathematics, 19.07.2019 02:30


Mathematics, 19.07.2019 02:30

Mathematics, 19.07.2019 02:30

Mathematics, 19.07.2019 02:30




Biology, 19.07.2019 02:30


English, 19.07.2019 02:30

Mathematics, 19.07.2019 02:30

English, 19.07.2019 02:30


Social Studies, 19.07.2019 02:30

Mathematics, 19.07.2019 02:30

Mathematics, 19.07.2019 02:30

Biology, 19.07.2019 02:30

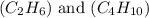 are 19.23 g and 20.41 g respectively.
are 19.23 g and 20.41 g respectively.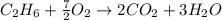
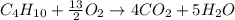
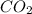 produced by burning each fuel is 1.28 mole and 1.41 mole respectively.
produced by burning each fuel is 1.28 mole and 1.41 mole respectively.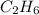
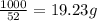
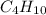 = 1 g
= 1 g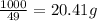
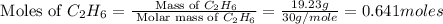
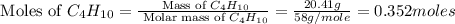
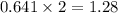 moles of
moles of 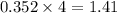 moles of
moles of 

