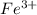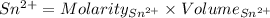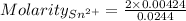Chemistry, 10.11.2019 04:31 xxtonixwilsonxx
Awire weighing 0.250 g and containing 94.75% fe is dissolved in hcl. the iron is completely oxidized to fe3+ by bromine water. the solution is then treated with tin(ii) chloride to bring about the reaction sn2+(aq) + 2fe3+(aq) → 2fe2+(aq) + sn4+(aq) + h2o(l) if 24.4 ml of tin(ii) chloride solution is required for complete reaction, what is the molarity of the tin(ii) chloride solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

You know the right answer?
Awire weighing 0.250 g and containing 94.75% fe is dissolved in hcl. the iron is completely oxidized...
Questions


Mathematics, 14.02.2020 05:11


Biology, 14.02.2020 05:11

English, 14.02.2020 05:12


Biology, 14.02.2020 05:12



Chemistry, 14.02.2020 05:12




Mathematics, 14.02.2020 05:12




Mathematics, 14.02.2020 05:13


Mathematics, 14.02.2020 05:13

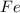 is 94.75 % by mass.
is 94.75 % by mass.
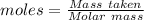
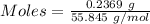
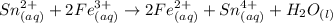
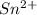 = 2*Moles of
= 2*Moles of