
Chemistry, 12.11.2019 02:31 rubincain203
Write a balanced equation for the combustion of c7h16(l) (heptane) -- i. e. its reaction with o2(g) forming the products co2(g) and h2o(l). given the following standard heats of formation: δhf° of co2(g) is -393.5 kj/mol δhf° of h2o(l) is -286 kj/mol δhf° of c7h16(l) is -187.8 kj/mol what is the standard heat of reaction (δh°) for the combustion reaction of c7h16(l)? -4854.7 kj calculate the difference, δh-δe=δ(pv) for the combustion reaction of 1 mole of heptane. (assume standard state conditions and 298 k for all reactants and products.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 07:30
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m.a. calculate the kc value for the a-protein binding reaction.b. calculate the kc value for the b-protein binding reaction.c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1
You know the right answer?
Write a balanced equation for the combustion of c7h16(l) (heptane) -- i. e. its reaction with o2(g)...
Questions



Computers and Technology, 26.03.2020 22:14


Social Studies, 26.03.2020 22:14




Geography, 26.03.2020 22:14



Mathematics, 26.03.2020 22:14

Mathematics, 26.03.2020 22:14

Mathematics, 26.03.2020 22:14

Mathematics, 26.03.2020 22:14

Mathematics, 26.03.2020 22:14


English, 26.03.2020 22:14


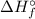 ) for: C₇H₁₆ (l) = -187.8 kJ/mol, O₂ (g) = 0 kJ/mol, CO₂ (g) = -393.5 kJ/mol, H₂O (l) = -286 kJ/mol
) for: C₇H₁₆ (l) = -187.8 kJ/mol, O₂ (g) = 0 kJ/mol, CO₂ (g) = -393.5 kJ/mol, H₂O (l) = -286 kJ/mol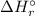 ) can be calculated by the Hess's law:
) can be calculated by the Hess's law:![\Delta H _{r}^{\circ } = \left [\sum \nu \cdot\Delta H _{f}^{\circ }(products) \right ] - \left [\sum \nu\cdot\Delta H _{f}^{\circ }(reactants) \right ]](/tpl/images/0369/9572/a156f.png)
 is the stoichiometric coefficient
is the stoichiometric coefficient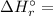
![\left [ 7\times \Delta H _{f}^{\circ }\left (CO_{2}\right )+ 8\times \Delta H _{f}^{\circ }\left (H_{2}O \right )\right ]](/tpl/images/0369/9572/3f95a.png)
![- \left [1\times \Delta H _{f}^{\circ }\left (C_{7}H_{16}\right ) +11\times \Delta H _{f}^{\circ }\left (O_{2} \right ) \right ]](/tpl/images/0369/9572/f3670.png)
![=\left [ 7\times \left (-393.5 kJ/mol \right )+ 8\times \left (-286 kJ/mol \right )\right ]](/tpl/images/0369/9572/7a112.png)
![-\left [1\times \left (-187.8 kJ/mol \right ) +11\times \left (0 kJ/mol \right ) \right ]](/tpl/images/0369/9572/be2b2.png)
![\Delta H _{r}^{\circ } = \left [ \left (-2754.5 \right )+ \left (-2288 \right )\right ]\left -[ \left (-187.8 \right ) +\left (0 \right )\right ]](/tpl/images/0369/9572/e3fd5.png)
![\Delta H _{r}^{\circ } = \left [ -5042.5 ]\left -[ -187.8] = \left ( -4854.7kJ \right )](/tpl/images/0369/9572/c52a2.png)


