
Chemistry, 12.11.2019 22:31 sydneykated
Write the reaction rate expressions for the reaction below in terms of the disappearance of the reactants and the appearance of products. give the expressions for the disappearance of the reactants first, in the order written in the chemical equation. then write the expressions for the appearance of the products in the order written in the chemical equation. write the expressions in order of appearance in the equation in the form. ± 1 x × δ[α] δt where ± is either a plus or a minus sign, not both, x is an integer, and a is a chemical species. do not include the state of matter. 2h2(g) + o2(g) → 2h2o(g)

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Write the reaction rate expressions for the reaction below in terms of the disappearance of the reac...
Questions


English, 26.12.2020 14:00

History, 26.12.2020 14:00


Arts, 26.12.2020 14:00

Mathematics, 26.12.2020 14:00

Social Studies, 26.12.2020 14:00

Business, 26.12.2020 14:00

Mathematics, 26.12.2020 14:00

English, 26.12.2020 14:00


Mathematics, 26.12.2020 14:00


Health, 26.12.2020 14:00



Mathematics, 26.12.2020 14:00

Chemistry, 26.12.2020 14:00


Mathematics, 26.12.2020 14:00

![-\frac{1d[H_2]}{2dt}](/tpl/images/0371/2323/5d767.png) rate of disappearance of oxygen =
rate of disappearance of oxygen = ![-\frac{1d[O_2]}{1dt}](/tpl/images/0371/2323/77c77.png)
![+\frac{1d[H_2O]}{2dt}](/tpl/images/0371/2323/aa544.png)
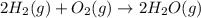
![Rate=k[H_2]^2[O_2]^1](/tpl/images/0371/2323/df759.png)


