Chemistry, 13.11.2019 02:31 tjjjjjjjjjjjjjjjjjjj
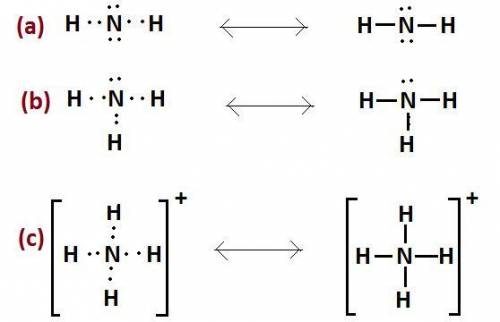
The nitrogen atom of nh2 would have the nitrogen atom of {\rm nh_2} would have blank electrons around the central nitrogen atom. electrons around the central nitrogen atom. the nitrogen atom of nh4 would have the nitrogen atom of {\rm nh_4} would have blank electrons around the central nitrogen atom. electrons around the central nitrogen atom. the nitrogen atom of nh3 would have the nitrogen atom of {\rm nh_3} would have blank electrons around the central nitrogen atom. electrons around the central nitrogen atom.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
The nitrogen atom of nh2 would have the nitrogen atom of {\rm nh_2} would have blank electrons aroun...
Questions

Mathematics, 28.05.2020 01:58




English, 28.05.2020 01:58


English, 28.05.2020 01:58


English, 28.05.2020 01:58


Physics, 28.05.2020 01:58




Mathematics, 28.05.2020 01:58


Physics, 28.05.2020 01:58



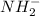 would have 8 electrons around the central nitrogen atom.
would have 8 electrons around the central nitrogen atom.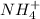 would have 8 electrons around the central nitrogen atom.
would have 8 electrons around the central nitrogen atom.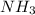 would have 8 electrons around the central nitrogen atom.
would have 8 electrons around the central nitrogen atom.