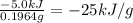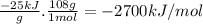Chemistry, 13.11.2019 21:31 joooosshhhh
Quinine is an important type of molecule that is involved in photosynthesis. the transport of electrons mediated by quinone in certain enzymes allows plants to take water, carbon dioxide, and the energy of sunlight to create glucose. a 0.1964-g sample of quinone (c6h4o2) is burned in a bomb calorimeter with a heat capacity of 1.56kj/c. the temperature of the calorimeter increases by 3.2 degrees c. calculate the energy of combustion of quinone per gram and per mole.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 1

You know the right answer?
Quinine is an important type of molecule that is involved in photosynthesis. the transport of electr...
Questions

Chemistry, 26.05.2021 01:30

Mathematics, 26.05.2021 01:30

Physics, 26.05.2021 01:30




Mathematics, 26.05.2021 01:30


Biology, 26.05.2021 01:30

Mathematics, 26.05.2021 01:30

Mathematics, 26.05.2021 01:30


Chemistry, 26.05.2021 01:30




English, 26.05.2021 01:30


Business, 26.05.2021 01:30

Mathematics, 26.05.2021 01:30