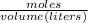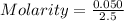Chemistry, 14.11.2019 23:31 menagirl71953
Then, calculate the molarity of a solution made by adding 5.57 g of cacl2 to enough water to create a solution with a total volume of 2.50 l. enter only a numerical value in the correct number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Then, calculate the molarity of a solution made by adding 5.57 g of cacl2 to enough water to create...
Questions
















Chemistry, 06.07.2019 05:20


English, 06.07.2019 05:20


Mathematics, 06.07.2019 05:20