
To melt the ice on your driveway, you can use two moles of rock salt (nacl) or two moles of calcium chloride (cacl2). which solute will have the greatest effect and why?
a) nacl because it is completely soluble in water.
b) nacl because it produces fewer moles of solute particles per mole of compound.
c) cacl2 because it produces more moles of solute particles per mole of compound.
d) cacl2 because it is less volatile than nacl.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1


Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

You know the right answer?
To melt the ice on your driveway, you can use two moles of rock salt (nacl) or two moles of calcium...
Questions


Spanish, 27.06.2019 02:00

English, 27.06.2019 02:00

English, 27.06.2019 02:00

History, 27.06.2019 02:00

Mathematics, 27.06.2019 02:00






English, 27.06.2019 02:00




Business, 27.06.2019 02:00




Chemistry, 27.06.2019 02:00

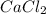 because it produces more moles of solute particles per mole of compound.
because it produces more moles of solute particles per mole of compound.

