Which of the following are isoelectronic?
a. k⁺¹, na⁺¹, he
b. mg⁺², o⁻², kr
c. n⁻...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1

You know the right answer?
Questions


History, 29.03.2020 01:30




Mathematics, 29.03.2020 01:30




Chemistry, 29.03.2020 01:30

Mathematics, 29.03.2020 01:30

Health, 29.03.2020 01:30

Physics, 29.03.2020 01:31


Mathematics, 29.03.2020 01:31

History, 29.03.2020 01:31


Social Studies, 29.03.2020 01:31

Mathematics, 29.03.2020 01:31

Mathematics, 29.03.2020 01:31

![[N]=1s^22s^22p^3](/tpl/images/0376/2884/27394.png)
![[N]^{3-}=1s^22s^22p^6](/tpl/images/0376/2884/17dd5.png)
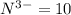
![[O]=1s^22s^22p^4](/tpl/images/0376/2884/336ff.png)
![[O]^{2-}=1s^22s^22p^6](/tpl/images/0376/2884/cd710.png)
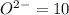
![[Ne]=1s^22s^22p^6](/tpl/images/0376/2884/5b2f7.png)
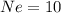
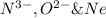 are isoelectronic.
are isoelectronic.

