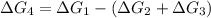Chemistry, 16.11.2019 00:31 dianasmygova
In nature, one common strategy to make thermodynamically unfavorable reactions proceed is to couple them chemically to reactions that are thermodynamically favorable. as long as the overall reaction is thermodynamically favorable, even the unfavorable reaction will proceed.
part a: consider these hypothetical chemical reactions:
a⇌b,δg= 11.9 kj/mol
b⇌c,δg= -26.7 kj/mol
c⇌d,δg= 7.30 kj/mol
what is the free energy, δg, for the overall reaction, a⇌d?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
In nature, one common strategy to make thermodynamically unfavorable reactions proceed is to couple...
Questions

History, 01.08.2020 08:01






Chemistry, 01.08.2020 08:01


Biology, 01.08.2020 08:01



Chemistry, 01.08.2020 09:01

Social Studies, 01.08.2020 09:01







 = 11.9 kJ/mol ...[1]
= 11.9 kJ/mol ...[1] = -26.7 kJ/mol ...[2]
= -26.7 kJ/mol ...[2]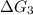 = 7.30 kJ/mol ...[3]
= 7.30 kJ/mol ...[3] = ?...[4]
= ?...[4]