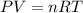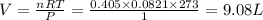Chemistry, 01.08.2020 08:01 alexis9263
4 Al + 3O2 → 2Al2O3 If 14.6 grams Al are reacted, how many liters of O2 at STP would be required?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
4 Al + 3O2 → 2Al2O3 If 14.6 grams Al are reacted, how many liters of O2 at STP would be required?...
Questions







Business, 27.11.2019 04:31













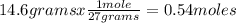
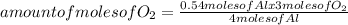
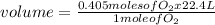
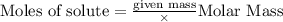
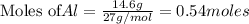
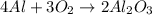
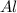 require = 3 moles of
require = 3 moles of 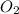
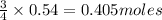 of
of