
Chemistry, 18.11.2019 19:31 mixedgirlmara
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6 kj / mol rxn. using the following standard enthalpy of formation data, calculate standard enthalpy of formation for sic (s). a. standard enthalpy of formation sio2 (s) = -910.9 kj/mol b. standard enthalpy of formation co (g) = -110.5 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6...
Questions


History, 28.01.2021 19:30

Mathematics, 28.01.2021 19:30


Chemistry, 28.01.2021 19:30

English, 28.01.2021 19:30


Mathematics, 28.01.2021 19:30



Mathematics, 28.01.2021 19:30

History, 28.01.2021 19:30

Mathematics, 28.01.2021 19:30

Mathematics, 28.01.2021 19:30

History, 28.01.2021 19:30

Mathematics, 28.01.2021 19:30

History, 28.01.2021 19:30


Mathematics, 28.01.2021 19:30

Mathematics, 28.01.2021 19:30

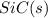 is coming out to be -65.3 kJ/mol
is coming out to be -65.3 kJ/mol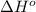
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0379/7554/72c39.png)
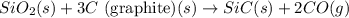
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times \Delta H^o_f_{(CO(g))})]-[(1\times \Delta H^o_f_{(SiO_2(s))})+(3\times \Delta H^o_f_{(C(s))})]](/tpl/images/0379/7554/6a7fe.png)
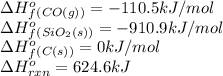
![624.6=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times (-110.5))]-[(1\times (-910.9))+(3\times (0))]\\\\\Delta H^o_f_{(SiC(s))}=-65.3kJ/mol](/tpl/images/0379/7554/c8e18.png)


