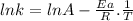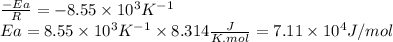The rate constant of a reaction is measured at different temperatures. a plot of the natural log of the rate constant as a function of the inverse of the temperature (in kelvins) yields a straight line with a slope of −8.55×103 k−1. what is the activation energy (ea) for the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2


You know the right answer?
The rate constant of a reaction is measured at different temperatures. a plot of the natural log of...
Questions


Computers and Technology, 21.07.2019 14:00

Mathematics, 21.07.2019 14:00

History, 21.07.2019 14:00




SAT, 21.07.2019 14:00




Arts, 21.07.2019 14:00


Mathematics, 21.07.2019 14:00


History, 21.07.2019 14:00


Mathematics, 21.07.2019 14:00