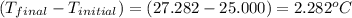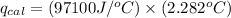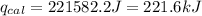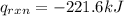Chemistry, 21.11.2019 01:31 Deadpool9609
4.41 g of propane gas (c3h8) is injected into a bomb calorimeter and ignited with excess oxygen, according to the reaction below. the calorimeter (including the water) has a heat capacity of 97.1 kj/°c. c3h8(g) + 5 o2(g) 3 co2(g) + 4 h2o() (a) if the temperature rose from 25.000°c to 27.282°c, what is the heat of the reaction, qrxn?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
4.41 g of propane gas (c3h8) is injected into a bomb calorimeter and ignited with excess oxygen, acc...
Questions

Chemistry, 20.05.2021 14:00

Chemistry, 20.05.2021 14:00

English, 20.05.2021 14:00


English, 20.05.2021 14:00



Physics, 20.05.2021 14:00


Computers and Technology, 20.05.2021 14:00






Mathematics, 20.05.2021 14:00


Physics, 20.05.2021 14:00

English, 20.05.2021 14:00

English, 20.05.2021 14:00

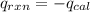
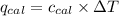
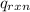 = heat released by the reaction = ?
= heat released by the reaction = ?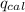 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter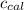 = specific heat of calorimeter =
= specific heat of calorimeter = 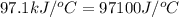
 = change in temperature =
= change in temperature =