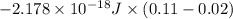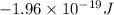Chemistry, 21.11.2019 20:31 jillianbarnes2565
An electron in the 7th energy level of the h atom drops to the 3th energy level. in other words an electron in an excited state drops to a less excited state. what is the energy (in j) of the emitted photon? the energy of an electron in the nth level

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

You know the right answer?
An electron in the 7th energy level of the h atom drops to the 3th energy level. in other words an e...
Questions

Mathematics, 19.10.2021 23:50


Mathematics, 19.10.2021 23:50

Mathematics, 19.10.2021 23:50



Mathematics, 19.10.2021 23:50


English, 19.10.2021 23:50

Mathematics, 19.10.2021 23:50

History, 19.10.2021 23:50

Mathematics, 19.10.2021 23:50

Social Studies, 19.10.2021 23:50

History, 19.10.2021 23:50


English, 19.10.2021 23:50

Spanish, 19.10.2021 23:50


World Languages, 19.10.2021 23:50

Mathematics, 19.10.2021 23:50

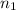 = 7,
= 7, 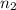 = 3
= 3![\Delta E = -2.178 \times 10^{-18} J \times (Z)^{2}[\frac{1}{n^{2}_{2}} - \frac{1}{n^{2}_{1}}]](/tpl/images/0384/9623/e92a6.png)
![-2.178 \times 10^{-18} J \times (1)^{2}[\frac{1}{(3)^{2}} - \frac{1}{(7)^{2}}]](/tpl/images/0384/9623/c0081.png)