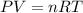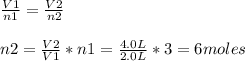Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3

Chemistry, 23.06.2019 11:30
Which of the following is a property of nonmetals? a.nonmetals are ductile. b.nonmetals have a shiny luster. c.nonmetals have high density. d.nonmetals are nonconductors.
Answers: 1
You know the right answer?
Assuming constant pressure and temperature, how many moles of gas have been added to the initial 3 m...
Questions

Biology, 12.12.2019 03:31

Mathematics, 12.12.2019 03:31




Mathematics, 12.12.2019 03:31

Mathematics, 12.12.2019 03:31

Mathematics, 12.12.2019 03:31

Mathematics, 12.12.2019 03:31

Mathematics, 12.12.2019 03:31


English, 12.12.2019 03:31

History, 12.12.2019 03:31


Business, 12.12.2019 03:31


Mathematics, 12.12.2019 03:31