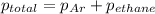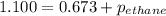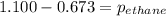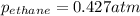Chemistry, 26.11.2019 06:31 Aviannakelly
A1.00 l flask is filled with 1.10 g of argon at 25 ∘c. a sample of ethane vapor is added to the same flask until the total pressure is 1.100 atm .1. part awhat is the partial pressure of argon, par, in the flask? express your answer to three significant figures and include the appropriate units.2. part bwhat is the partial pressure of ethane, pethane, in the flask? express your answer to three significant figures and include the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
A1.00 l flask is filled with 1.10 g of argon at 25 ∘c. a sample of ethane vapor is added to the same...
Questions




Biology, 25.10.2019 17:43

Biology, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43

World Languages, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43





Mathematics, 25.10.2019 17:43


Biology, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43