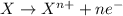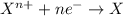Chemistry, 27.11.2019 01:31 slbucknerholmes9
23. metal corrosion is a reaction during which a metal becomes oxidized. which of the statements below are true about this kind of reaction? check all that apply.
a) if oxygen is involved, it supplies electrons to the metal.
b) the oxidation state of the metal increases (its oxidation number becomes more positive).
c) the oxidation process involves loss of electrons by the metal.
d) there must be direct contact between the metal and oxidizer for electron transfer to take place.
e) another atom must be reduced as the metal is oxidized.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
23. metal corrosion is a reaction during which a metal becomes oxidized. which of the statements bel...
Questions

English, 01.07.2019 12:30

Mathematics, 01.07.2019 12:30

Mathematics, 01.07.2019 12:30




History, 01.07.2019 12:30


Mathematics, 01.07.2019 12:30

Mathematics, 01.07.2019 12:30


Mathematics, 01.07.2019 12:30


Mathematics, 01.07.2019 12:30


Mathematics, 01.07.2019 12:30

Mathematics, 01.07.2019 12:30

History, 01.07.2019 12:30

Health, 01.07.2019 12:30

Mathematics, 01.07.2019 12:30