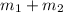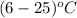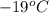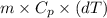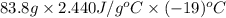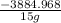When 15.0 g of a salt was dissolved in 68.8 g of ethyl alcohol, the temperature of the solution decreased from 25.0 °c to 6.0 °c. the specific heat capacity for ethyl alcohol is 2.440 j/g°c. calculate the heat of reaction (∆h) in units of kj/g. (equation 1 and equation 2 will be .)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
When 15.0 g of a salt was dissolved in 68.8 g of ethyl alcohol, the temperature of the solution decr...
Questions

Mathematics, 17.03.2020 17:32


Mathematics, 17.03.2020 17:32


Mathematics, 17.03.2020 17:32



Geography, 17.03.2020 17:32

Biology, 17.03.2020 17:33


Chemistry, 17.03.2020 17:33

Mathematics, 17.03.2020 17:33



Biology, 17.03.2020 17:33





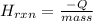
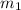 = 15 g of salt,
= 15 g of salt, 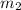 = 68.8 g of ethanol
= 68.8 g of ethanol