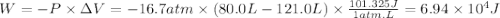Chemistry, 28.11.2019 05:31 loraine4664
Calculate the work associated with the compression of a gas from 121.0 l to 80.0 l at a constant pressure of 16.7 atm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Calculate the work associated with the compression of a gas from 121.0 l to 80.0 l at a constant pre...
Questions


Computers and Technology, 11.11.2019 20:31


Chemistry, 11.11.2019 20:31

Physics, 11.11.2019 20:31

Mathematics, 11.11.2019 20:31


History, 11.11.2019 20:31


Mathematics, 11.11.2019 20:31

English, 11.11.2019 20:31


Mathematics, 11.11.2019 20:31

History, 11.11.2019 20:31






Physics, 11.11.2019 20:31