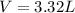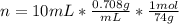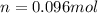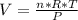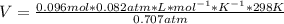Chemistry, 30.11.2019 02:31 Sparkleskeepsgoing
Diethyl ether, c4h10o was a widely used anesthetic in the early days of surgery. it has a vapor pressure of 537 torr at 25 oc. a 10.00 ml sample (d = 0.708 g/ml) is placed in a sealed 0.205 l flask. what is the maximum volume in liters the flask can have if equilibrium is to be maintained between liquid and vapor?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Diethyl ether, c4h10o was a widely used anesthetic in the early days of surgery. it has a vapor pres...
Questions


Physics, 26.03.2020 20:53




Mathematics, 26.03.2020 20:53



Mathematics, 26.03.2020 20:54


Biology, 26.03.2020 20:54

Biology, 26.03.2020 20:54


Mathematics, 26.03.2020 20:54


Mathematics, 26.03.2020 20:54


Mathematics, 26.03.2020 20:54