Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3


Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
The solubility of gold (iii) chloride is 1.00x10^-4 g/l. what is the ksp for aucl3 (molar mass=303.3...
Questions








Chemistry, 02.01.2020 20:31


Computers and Technology, 02.01.2020 20:31



Computers and Technology, 02.01.2020 20:31






Health, 02.01.2020 20:31

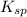 of gold (III) chloride is
of gold (III) chloride is 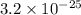
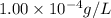
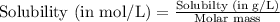
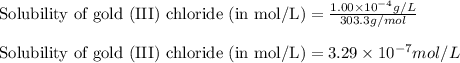
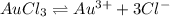
![K_{sp}=[Au^{3+}][Cl^-]^3](/tpl/images/0399/6764/11923.png)