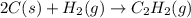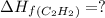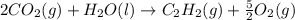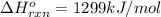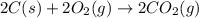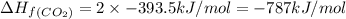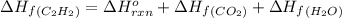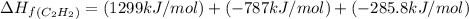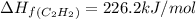The enthalpy of combustion of acetylene c2h2 is described by
c2h2 (g) + (5/2)o2 (g) >...

The enthalpy of combustion of acetylene c2h2 is described by
c2h2 (g) + (5/2)o2 (g) > > > > > > > co2 (g) + h2o (l) heat of reaction (rxn) = -1299kj/mol
calculate the enthalpy of formation of accetylene, given the following enthalpies of formation
standard formation [co2 (g)]= -393.5 kj/mol
standard formation [h2o (l)] = -285.8 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
Questions



Chemistry, 31.08.2019 03:30

Chemistry, 31.08.2019 03:30







English, 31.08.2019 03:30


Mathematics, 31.08.2019 03:30



Mathematics, 31.08.2019 03:30

Chemistry, 31.08.2019 03:30


Mathematics, 31.08.2019 03:30

English, 31.08.2019 03:30

 will be,
will be,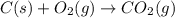
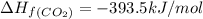
 will be,
will be,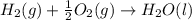
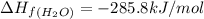
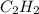 will be,
will be,