
Chemistry, 03.12.2019 21:31 courtnicollee
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the solution, which converts fe(ii) to insoluble fe(iii): 4fe(oh)+(aq)+ 4oh-(aq)+o2(g)+2h2o(l)=4fe(oh)3(s)h ow many grams of o2 are consumed to precipitate all of the iron in 85ml of 0.075 m fe(ii).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the soluti...
Questions




Computers and Technology, 27.11.2019 05:31
















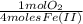 = 1,6x10⁻³ moles of O₂(g)
= 1,6x10⁻³ moles of O₂(g)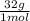 = 0,051g of O₂ are consumed
= 0,051g of O₂ are consumed

