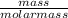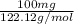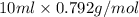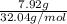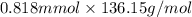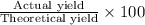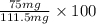Chemistry, 04.12.2019 00:31 Aydenj9613
Assume that you react 100 mg of benzoic acid with 10 ml of methanol and 10 microliters of sulfuric acid to produce methyl benzoate. write a balance chemical equation for this reaction. determine the limiting reagent and calculate a theoretical yield of both the ester and water. if you isolate 75 mg of methyl benzoate, what is the actual yield of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
Assume that you react 100 mg of benzoic acid with 10 ml of methanol and 10 microliters of sulfuric a...
Questions






Mathematics, 26.02.2021 07:10

English, 26.02.2021 07:10

Biology, 26.02.2021 07:10

Computers and Technology, 26.02.2021 07:10

Physics, 26.02.2021 07:10


Mathematics, 26.02.2021 07:10

Mathematics, 26.02.2021 07:10

Mathematics, 26.02.2021 07:10


Mathematics, 26.02.2021 07:10

Mathematics, 26.02.2021 07:10

Mathematics, 26.02.2021 07:10

Business, 26.02.2021 07:10

Mathematics, 26.02.2021 07:10

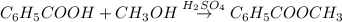
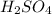 is very small so, that is catalytic amount of
is very small so, that is catalytic amount of