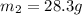Chemistry, 04.12.2019 02:31 kayleahrayne
Asample of nickel is heated to 99.8 degrees c and placed in a coffee cup calorimeter containing 150.0g water at 23.5 degrees c. after the metal cools, the final temperature of metal andwater mixture is 25.0 degrees c. if the specific heat capacity of nickel is 0.444j/(degreescg),
what mass of nickel was originally heated?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1


Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

You know the right answer?
Asample of nickel is heated to 99.8 degrees c and placed in a coffee cup calorimeter containing 150....
Questions


Mathematics, 16.07.2021 19:20


Biology, 16.07.2021 19:20


Mathematics, 16.07.2021 19:20



Physics, 16.07.2021 19:20

Biology, 16.07.2021 19:20


Mathematics, 16.07.2021 19:20

Mathematics, 16.07.2021 19:20

Mathematics, 16.07.2021 19:20


History, 16.07.2021 19:20



Physics, 16.07.2021 19:20

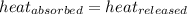
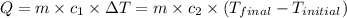
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0402/0900/09236.png) .................(1)
.................(1)
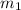 = mass of water= 150.0 g
= mass of water= 150.0 g
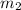 = mass of nickel = ?
= mass of nickel = ?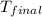 = final temperature =
= final temperature = 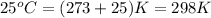
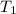 = temperature of water =
= temperature of water = 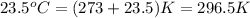
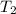 = temperature of nickel =
= temperature of nickel = 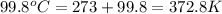
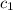 = specific heat of water =
= specific heat of water = 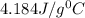
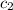 = specific heat of nickel=
= specific heat of nickel= 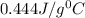
![150.0\times 4.184\times (298-296.5)=-[m_2\times 0.444\times (298-372.8)]](/tpl/images/0402/0900/54914.png)