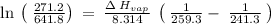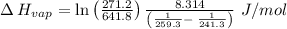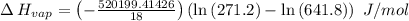Chemistry, 04.12.2019 03:31 gracynamos
The vapor pressure of the liquid so2 is measure at different temperatures. the following vapor pressure data are obtained. temp (k) pressure mmhg241.3 271.2259.3 641.8calculate the enthalpy of vaporization(delta h vap) in kj/mol for this liquid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
The vapor pressure of the liquid so2 is measure at different temperatures. the following vapor press...
Questions



Spanish, 11.01.2021 22:00

Chemistry, 11.01.2021 22:00


Mathematics, 11.01.2021 22:00




Arts, 11.01.2021 22:00


Chemistry, 11.01.2021 22:00

History, 11.01.2021 22:00

Mathematics, 11.01.2021 22:00

Physics, 11.01.2021 22:00

History, 11.01.2021 22:00



Computers and Technology, 11.01.2021 22:00

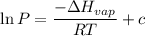
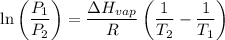
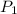 = 271.2 mmHg
= 271.2 mmHg
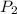 = 641.8 mmHg
= 641.8 mmHg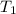 = 241.3 K
= 241.3 K 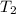 = 259.3 K
= 259.3 K