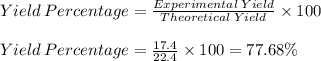Chemistry, 05.12.2019 08:31 LilFlamingo247
Consider the balanced chemical reaction below and determine the percent yield for iron if 11.2 moles of iron(iii) oxide yielded 17.4 moles of iron
fe2o3+3co> 2fe+3co2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
Consider the balanced chemical reaction below and determine the percent yield for iron if 11.2 moles...
Questions



Chemistry, 18.03.2021 01:20




Health, 18.03.2021 01:20




Social Studies, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Chemistry, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20



Mathematics, 18.03.2021 01:20



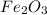 yields 2 moles of Fe.
yields 2 moles of Fe.