
Chemistry, 05.12.2019 22:31 cuthbertson157
Dissolving 5.28 g of an impure sample of calcium carbonate in hydrochloric acid produced 1.14 l of carbon dioxide at 20.0 â°c and 791 mmhg. calculate the percent by mass of calcium carbonate in the sample.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Dissolving 5.28 g of an impure sample of calcium carbonate in hydrochloric acid produced 1.14 l of c...
Questions


Chemistry, 08.12.2020 02:10

Biology, 08.12.2020 02:10


Mathematics, 08.12.2020 02:10


Social Studies, 08.12.2020 02:10

Mathematics, 08.12.2020 02:10



Health, 08.12.2020 02:10

English, 08.12.2020 02:10


Mathematics, 08.12.2020 02:10

Physics, 08.12.2020 02:10

Mathematics, 08.12.2020 02:10




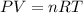
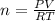
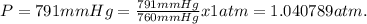
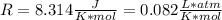
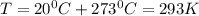
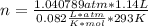
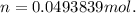
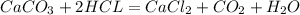
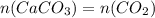
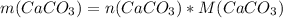
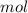 * 100
* 100 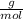 = 4.93839
= 4.93839 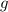
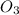 =
= 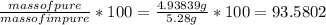 .
.

