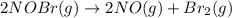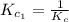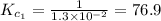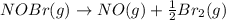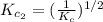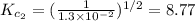Chemistry, 06.12.2019 02:31 taylorb9893
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at this temperature does the equilibrium favor no and br2, or does it favor nobr? calculate kc for 2nobr(g)⥫⥬==2no(g)+br2(g). calculate kc for nobr(g)⥫⥬==no(g)+12br2(g).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at thi...
Questions

English, 03.12.2020 17:20

Mathematics, 03.12.2020 17:20

Physics, 03.12.2020 17:20


Health, 03.12.2020 17:20






English, 03.12.2020 17:20


Mathematics, 03.12.2020 17:20

Arts, 03.12.2020 17:20






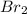 .
.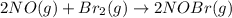
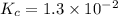 .
.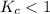 that means equilibrium lies to the left side. Thus, the equilibrium favors NO and
that means equilibrium lies to the left side. Thus, the equilibrium favors NO and