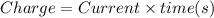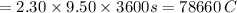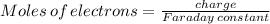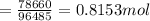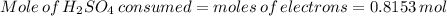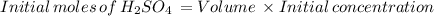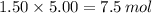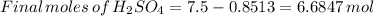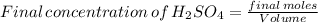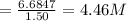Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Suppose that a fully charged lead-acid battery contains 1.50 l of 5.00 m h2so4. what will be the con...
Questions

Computers and Technology, 05.12.2019 06:31



Mathematics, 05.12.2019 06:31








Mathematics, 05.12.2019 06:31

Mathematics, 05.12.2019 06:31

History, 05.12.2019 06:31