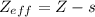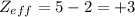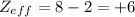Chemistry, 06.12.2019 20:31 jadepotts3965
The shielding of electrons gives rise to an effective nuclear charge,  , which explains why boron is larger than oxygen. estimate the approximate
, which explains why boron is larger than oxygen. estimate the approximate  felt by a valence electron of boron and oxygen, respectively? a. +5 and +8b. +3 and +6c. +5 and +6d. +3 and +8e. +1 and +4
felt by a valence electron of boron and oxygen, respectively? a. +5 and +8b. +3 and +6c. +5 and +6d. +3 and +8e. +1 and +4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2


Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
The shielding of electrons gives rise to an effective nuclear charge, [tex]z_{eff}[/tex], which expl...
Questions

Spanish, 25.02.2021 01:00







History, 25.02.2021 01:00


Mathematics, 25.02.2021 01:00

Chemistry, 25.02.2021 01:00



Mathematics, 25.02.2021 01:00

Mathematics, 25.02.2021 01:00

Mathematics, 25.02.2021 01:00



Mathematics, 25.02.2021 01:00

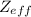 of the valence electron of boron and oxygen, we need to use the next equation:
of the valence electron of boron and oxygen, we need to use the next equation: