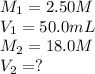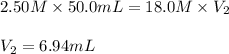Chemistry, 07.12.2019 00:31 juniorvaldez60
In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only available stock solution of the acid has a concentration of 18.0 m. what volume of the stock solution would they use to make the required solutiona. 0.900 ml
b. 1.11 ml
c. 6.94 ml
d. 7.20 ml

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only a...
Questions

Mathematics, 18.02.2021 08:30

Health, 18.02.2021 08:30


Mathematics, 18.02.2021 08:30

Health, 18.02.2021 08:30

Arts, 18.02.2021 08:30


Mathematics, 18.02.2021 08:30



Mathematics, 18.02.2021 08:30

Mathematics, 18.02.2021 08:30

Mathematics, 18.02.2021 08:30



Mathematics, 18.02.2021 08:30


Geography, 18.02.2021 08:30

Mathematics, 18.02.2021 08:30

Biology, 18.02.2021 08:30

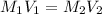
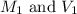 are the molarity and volume of acid which is
are the molarity and volume of acid which is 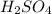
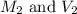 are the molarity and volume of stock solution of acid.
are the molarity and volume of stock solution of acid.