
In the simulation, open the custom mode. the beaker will be filled to the 0.50 l mark with a neutral solution. set the ph to 11.30 by using the green arrows adjacent to the ph value indicated on the probe in the solution. once you adjust the ph, note the corresponding oh− ion concentration in m as given in the graphic on the left side of the simulation. make sure to select the option "concentration (mol/l)" above the graphic. find the poh of the solution. express your answer numerically to two decimal places.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


You know the right answer?
In the simulation, open the custom mode. the beaker will be filled to the 0.50 l mark with a neutral...
Questions

Mathematics, 19.06.2021 08:20

Biology, 19.06.2021 08:20




Mathematics, 19.06.2021 08:20

Computers and Technology, 19.06.2021 08:20




Mathematics, 19.06.2021 08:20



Mathematics, 19.06.2021 08:30



Mathematics, 19.06.2021 08:30


Biology, 19.06.2021 08:30

Mathematics, 19.06.2021 08:30

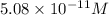 .
.![2.7= -log[OH^{-}]](/tpl/images/0407/3948/5748b.png)
![log[OH^{-}]= -2.7=5.08\times10^{-11}M](/tpl/images/0407/3948/7f9ac.png)


