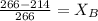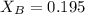Chemistry, 09.12.2019 18:31 angelb2472
The vapor pressure of pure acetone is 266 torr. when a non-volatile solute is added, the vapor pressure of acetone above the solution falls to 214 torr. what is the mole fraction of the non-volatile solute in the solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
The vapor pressure of pure acetone is 266 torr. when a non-volatile solute is added, the vapor press...
Questions

Mathematics, 03.12.2019 04:31


Mathematics, 03.12.2019 04:31

Biology, 03.12.2019 04:31





Mathematics, 03.12.2019 04:31


Mathematics, 03.12.2019 04:31




Mathematics, 03.12.2019 04:31

English, 03.12.2019 04:31

History, 03.12.2019 04:31


Health, 03.12.2019 04:31

Mathematics, 03.12.2019 04:31

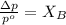
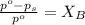
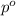 = vapor pressure of the pure solvent (acetone) = 266 torr
= vapor pressure of the pure solvent (acetone) = 266 torr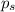 = vapor pressure of the solution = 214 torr
= vapor pressure of the solution = 214 torr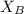 = mole fraction of solute = ?
= mole fraction of solute = ?