
Consider two flasks at 25 degree celsius, one contains an ideal gas and the other contains the real gas so3. which statement regarding these gases is true?
a. as the temperature approaches 0 k, the volume of the ideal gas will be larger than the volume of so3 because ideal gases lack inter-molecular forces.
b. as the temperature is decreased, the ideal gas will liquefy first because ideal gases have larger values of the van der waals coefficient b.
c. as the temperature approaches 0 k, the volume of the ideal gas will be smaller than the volume of so3 because ideal gases have larger values of the van der waals coefficient a.
d. after the temperature has been lowered about 100 k, the pressure of the ideal gas will be smaller than the pressure of so3 because the van der waals coefficient a for so3 is large.
e. after the temperature is increased about 100 k, the pressure of the ideal gas will be smaller than the pressure of so3 because the van der waals coefficient a for so3 is lar

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
Consider two flasks at 25 degree celsius, one contains an ideal gas and the other contains the real...
Questions





Computers and Technology, 07.04.2020 05:00







Mathematics, 07.04.2020 05:00


Biology, 07.04.2020 05:00



Computers and Technology, 07.04.2020 05:00


Mathematics, 07.04.2020 05:00

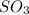 because ideal gases lack inter-molecular forces, is true.
because ideal gases lack inter-molecular forces, is true.

