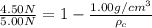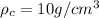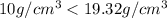Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
Imagine that the apparent weight of the crown in water is [tex]w_{apparent}[/tex] = 4.50n, and the a...
Questions


Mathematics, 12.03.2020 06:43



Mathematics, 12.03.2020 06:43

History, 12.03.2020 06:43




Mathematics, 12.03.2020 06:43


Business, 12.03.2020 06:44


Computers and Technology, 12.03.2020 06:44

Mathematics, 12.03.2020 06:44





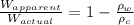
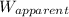 = apparent weight of the crown in water = 4.50 N
= apparent weight of the crown in water = 4.50 N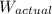 = actual weight = 5.00 N
= actual weight = 5.00 N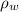 = density of water =
= density of water = 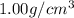
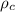 = density of crown = ?
= density of crown = ?